- Home » Editorial » Hydraulics
Reducing cross contamination in pharma applications
By Matt Hale, International Sales and Marketing Director, HRS Heat Exchangers.
Cross contamination in the pharmaceutical industry is not just an issue for those working in it, but for the wider public as a whole. As general awareness of the potential issues has increased, more and more questions are being asked of manufacturers and health professionals about the safety, integrity and potential side effects of a range of products and medicines.
Protecting people and planet
Understandably, most of the attention on contamination in pharma manufacturing and processing is focused on either cross contamination between products or agents, or from the spread of agents, such as biological cultures, within a facility. In fact, the potential harm of biological and other agents escaping from production sites has recently been highlighted by environmental pollution from antibiotics in a number of countries, which is believed to be a contributing factor to rising levels of antibiotic resistance around the world1. Furthermore, viral contamination can also be a serious threat for any type of bioprocessing laboratory or manufacturing facility2.
Ensuring brand integrity
Thanks to the increasing use of outsourced production, new technologies and continually evolving biological and microbiological techniques, ensuring safe pharma manufacturing has become increasingly complex3. However, designers, manufacturers and operators need to take concerns seriously as even relatively small issues can quickly escalate, not only in terms of the actual effects they may have on consumers, clients and the environment, but also the potential damage they can cause to company reputation and valuable brands.
A healthy and hygienic workspace
Attention also needs to be given to the potential contamination of (and from) environmental factors used in the production and processing of pharmaceuticals, such as water and air used for process heating, cooling or sterilisation. Obviously, the best option is to prevent contamination in the first place and there are many regulations to enforce this.
For example, pharmaceutical, cosmetic and medical ingredients often have very tight temperature tolerances, requiring specific operations to be carried out at specific temperatures, while applications such as the production of Purified Water and Water for Injection (WFI) require the highest standards of hygiene. However, many of today’s pharmaceutical manufacturers are going above and beyond this, reducing their contamination risk further through the use of robust procedures and well-designed equipment.
Pharma-ready heat exchangers
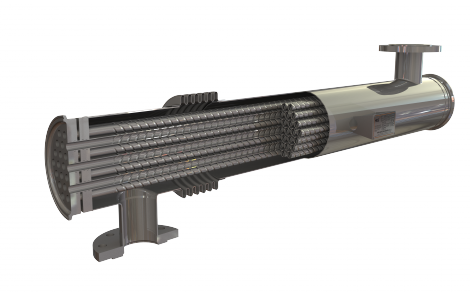
One such example is the new and improved HRS S Series of corrugated multi-tube heat exchangers. The S Series is fitted with a double tube plate to overcome the potential issue of contamination between the product side and service side materials which is a prerequisite for some pharmaceutical applications. This design prevents contamination and provides leak detection while offering all the usual benefits of HRS Heat Exchangers’ normal corrugated tube design, including reduced fouling, improved heat transfer and reduced pressure drop. All S Series models also feature an expansion bellows to absorb different expansion rates between the shell and inner tubes. Individual units are available with surface areas from 1.3 to 6.8 m2, and units can be combined in a frame for larger applications. Surface finishes range from 0.4 to 0.8μ for pharmaceutical and hygienic industries and descaled for all industrial applications.
-
PPMA 2025
23 September, 2025, 9:30 - 25 September, 2025, 16:00
NEC, Birmingham UK -
Advanced Engineering Show 2025
29 October, 2025, 9:00 - 30 October, 2025, 16:00
NEC, Birmingham UK










